Search This Supplers Products:Magnetic materials, magnetic equipment and devicesAuto Partstelecom devicesElectronicselectricalsmetals
Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer
time2020/09/10
- Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer
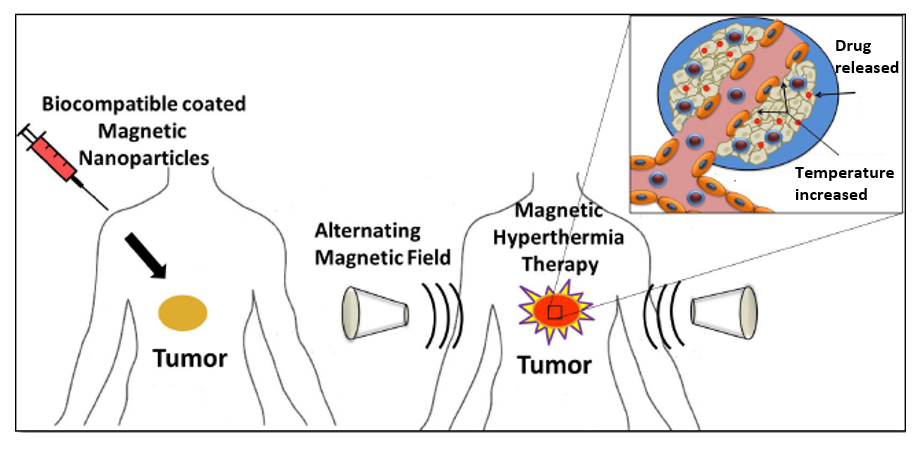
Cancer has become one of the major public health concerns in our modern society, resulting in the death of more than 8.8 million people every year across the world in 2015. Magnetic hyperthermia treatment (MHT) is one of the promising non-invasive approaches for thermal activation therapy on cancerous tumors. Upon exposure to external alternating current magnetic fields, MNPs can generate heat through the oscillation of their magnetic moments. MHT can then kill cancer cells by elevating their temperature to 40°C-45°C with minimal injury to normal cells. Magnetic Hyperthermia based heating systems could be employed either for the controlled release of a drug from caged drug delivery systems or the killing of tumor cells or both.

For hyperthermia, large variety of magnetic nanoparticles, magnetite (Fe3O4) and maghemite (γ-Fe2O3) are most widely used magnetic system for hyperthermia applications because of their selective heating capacity without damaging healthy tissues, superior bio-compatibility, ease of synthesis and long-term stability. Heart et al. Injected 100 mg dextran coated magnetic nanoparticles into the tail vein of Sprague Dawley rats, treated with AC magnetic field (12 min, 450 kHz, unknown field and SAR). According to authors’ considerable the tumor shrinkage and tissue necrosis was observed. The localized heat was achieved by subjecting (PVA+Fe3O4) ferrogel to alternating magnetic field and maximum temperature of 43°C was 2.5 wt.% Fe3O4 concentrations.
Maximum temperature can be easily adjusted by changing the Fe3O4 magnetic field strength so as meet different requirement of various cancer or tumor cells. reported the comparison of chitosan-coated magnetic nanoparticle with uncoated and starch-coated magnetic nanoparticles targeting to carcinoma cells in hyperthermia.
The chitosan-coated magnetic nanoparticles generated a higher ∆T of 23°C under an AC magnetic field than the starch coated magnetic nanoparticles and the capturing rate of the magnetic nanoparticles was also 96% under an external magnetic field of 0.4 T. The Fe3O4 magnetic nanoparticles prepared without capping (Fe3O4) and Fe3O4 capped with polyethylene glycol (PEG). The heating ability of Fe3O4 capped with PEG up to 42°C was observed at reasonably low concentrations, suggesting their suitability for hyperthermia applications.
The efficacy of capped Fe3O4 was further validated in human breast cancer cells with their enhanced killing ability under induction heating conditions suggesting the extension of these studies for hypothermic cancer therapy in suitable in vivo experimental models. The temperature rises under AC magnetic field, cytotoxicity. In vitro hyperthermia effect of Pluronic-coated Fe3O4 nanoparticles.