Search This Supplers Products:Magnetic materials, magnetic equipment and devicesAuto Partstelecom devicesElectronicselectricalsmetals
Why can it be picked up by a magnet after burning? The little mystery of the head of the match.
sourcebaijiahao
publisherGordon
time2013/12/10

- After the match burns, the remaining matchstick can be attracted by the magnet, which means they are magnetic. Generally speaking, after this kind of match is burnt, the stick will be thrown away as a waste. This is just a piece of wood, why is it attracted by the magnet? You must know that it must have iron content to be attracted by a magnet, and it is impossible for these woods to contain such an ingredient, right? So why is it directly attracted? What is the mystery in this?
After the match burns, the remaining matchstick can be attracted by the magnet, which means they are magnetic. After this kind of match is burnt, the stick will be thrown away as a waste. This is just a piece of wood, why is it attracted by the magnet? You must know that it must have iron content to be attracted by a magnet, and it is impossible for these woods to contain such an ingredient, right? So why is it directly attracted? What is the mystery in this?
Let's look at this match first. What are the components of the burning part? The burning components are composed of potassium chlorate, sulfur, and red phosphorus. However, after these are burned, they cannot form iron oxide that magnets attract, so these products will not cause this phenomenon. Therefore, just by looking at the composition of the top part, we cannot find the answer we want. Could it be that the products formed after they burn can also be attracted by the magnet? Of course, this answer is obviously wrong.
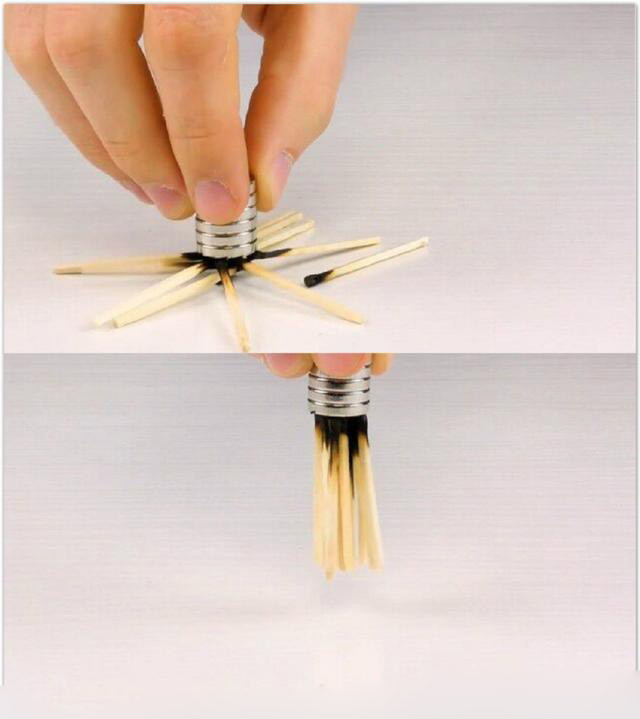
Then, in addition to the things we mentioned above, does the burning part of this match contain anything else? Many people may feel that there are no other ingredients. After all, this is a very common thing, and it is impossible to contain how advanced chemical ingredients are in it. Of course, what is said here is not particularly expensive or relatively rare. ingredient. Because everyone knows that the matchstick is made of wood, and there are some chemical components on it, then the two must be together to use adhesives, and we will find its head when we buy matches. There are different colors, most of them will show a dark red, of course, some colors are more vivid, then this coloring involves some chemical components.
Moreover, after the research scholars analyzed it, they found that the reason that caused the burned matchstick to be attracted by the magnet, and the answer lies in the color of the match, which means that the match was dyed reddish brown. In fact, the chemical composition used to dye this color is what we call ferric oxide.
Of course, this is not to say that these experiments were made up by those who did the experiment, because previous researchers also used some advanced instruments to analyze this, especially the use of proton x-ray fluorescence analysis. The results showed that it was indeed contained in the combustion. A kind of component of, and this component will have a certain reaction during the combustion process, so the final substance left is called ferro ferric oxide. Although it is said to have reacted, it is magnetic. Therefore, it is precisely because of this special composition that after the match is burned, even a small piece that looks like only wood can be magically attracted by the magnet.

Therefore, you must not think that this is a miraculous phenomenon. This is a chemical reaction. Because of this special composition, it can lead to being attracted.
Of course, if you buy more different matches on the market, there are some matches whose coloring is not iron oxide color, but other colors, such as some dyes and dyes. Other colors will probably not be sucked up after burning.
Researchers have also burned matches together with iron oxide and other components. After burning, they will be attracted. It is indeed because of the presence of iron oxide. Of course, this magnet should also have sufficient magnetic force. This is amazing.
